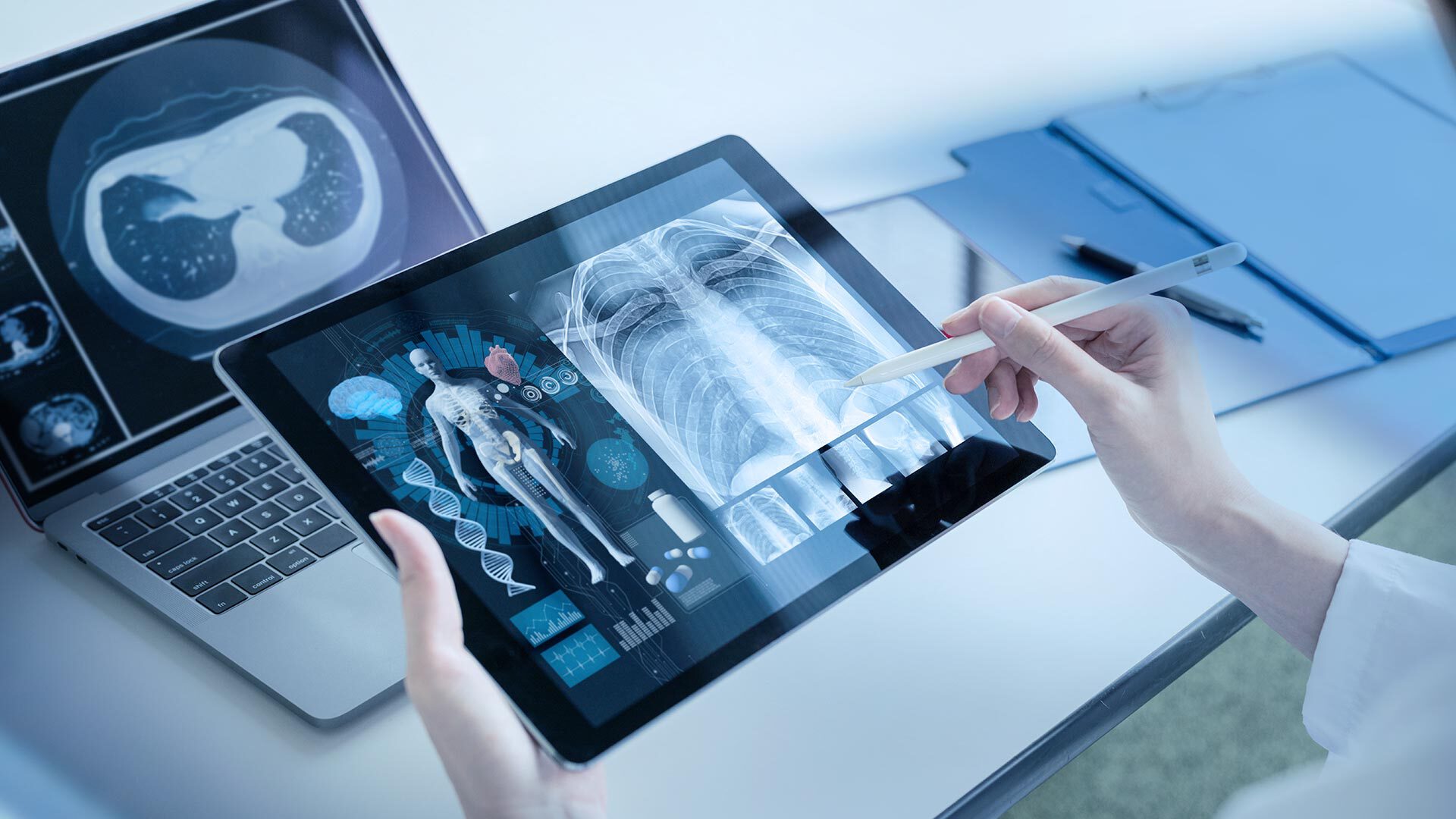
The field of medical technology is highly diverse and innovative. The number and performance of technologies used in medical devices has evolved steadily over the years. These devices are designed for use in a variety of situations and environments – thus they are subject to numerous standards and regulations.
For successful market access, these medical devices must meet basic safety and performance requirements, which can vary widely by market. That’s why we support our medical industry customers with various services regarding medical device testing and approval procedures. We enable the certification of their medical devices as well as their successful market launch.
cetecom advanced is taking part in this innovative research project together with 18 other companies.
We are at the beginning of an exciting era in the world of medical technology, a sector that is characterized by its diversity and innovative strength. As the technologies in medical devices continue to develop and become more powerful, new horizons are opening up for their use in a wide variety of situations and environments.
Over the next three years, cetecom advanced will be taking part in the innovative 6G Health research project – funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) – together with 18 other renowned companies. This project will investigate the development of the next generation of wireless communication in the medical technology and healthcare sector and its potential applications.

This project brings together companies and organizations in communications engineering and medical technology to enable tailored technology developments in the field of 6G wireless technology.
The 6G Health project focuses on the development of sixth-generation (6G) wireless networks. Due to its scalability in terms of bandwidth, range, energy efficiency and reliability, this technology offers a breeding ground for a wide range of applications for the healthcare sector, e.g. for the purpose of:
Dr. Matthias Flieger, head of our certification department, is representing cetecom advanced as project manager with the support of Dr. Nina Müller, Certification Manager in his team in this promising project, which is being carried out together with renowned partners from research, development and the service sector.
One of the main focuses of cetecom advanced’s activities is the development of compliance strategies for the subsequent market approval of innovative medical devices. At the current stage of the project, Dr. Matthias Flieger and Dr. Nina Müller are aiming for general acceptance of medical testing standards for wireless medical products. In collaboration with all project partners, the plan is to create a basis for a standardization recommendation that will ultimately provide manufacturers with planning security when introducing products.
The 6G Health project demonstrates the potential of 6G in the healthcare sector and paves the way for holistic optimization of healthcare across patients, healthcare staff and processes.
We are delighted to be part of this exciting project and look forward to taking the next steps together to discover and enable a new generation of mobile communication for the medical and healthcare sector.
Should you require further information on the 6G Health project, please do not hesitate to contact us: mail@cetecomadvanced.com.
With individually tailored consulting, testing and certification solutions, we ensure that medical devices meet the quality, health, environmental and safety standards required for market access. Our test reports are recognized worldwide and thus provide the foundation for market approval in over 180 target countries.
We hold DIN/ISO/IEC 17025 accreditation by DAkkS and are also recognized by ZLG (Central Coordination Office for Human and Veterinary Medicinal Products in Germany). With our broad testing portfolio and expertise, we enable fast and cost-effective medical device testing and approval of medical technology.
We have many years of experience regarding electrical safety and electromagnetic compatibility (EMC) testing.
As a recognized test service provider, we offer the following test procedures for medical devices / medical technology:
In addition, we are an accredited test laboratory for the CB scheme (CB Report) in electrical safety and EMC.
As a Partner Test Laboratory (PTL) we can also provide NRTL certifications through our network.
Medical devices that use wireless radio technologies, such as Bluetooth®, Wi-Fi, DECT, radar or NFC must meet the requirements of both the Medical Device Directive and the Radio Equipment Directive (2014/53/EU) in Europe.
The European Union’s Radio Equipment Directive (RED; replaced R&TTE in 2014) applies to electrical and electronic equipment that intentionally emits and/or receives radio waves. Medical devices with radio technologies thus also fall into this category.
Our testing services cover all common radio technologies according to current norms and standards. Thus, we can provide medical device testing services as well as the certification of medical technology to enable an early approval on the market.
In addition to regulatory testing and certification services, we also offer many other services for medical devices. These include: